Every tin plated copper alloy experiences the formation of copper-tin intermetallic compounds (Cu6Sn5 and Cu3Sn) at the interface of the tin and the base metal. [An intermetallic compound consists of two or more elements, always with the same precise ratio of atoms (in this case 6:5 and 3:1).] Many dissimilar metals (tin and copper) in close contact will diffuse and form intermetallic compounds. The compound initially forms at the interface of the plating and the base metal and grows until eventually all the tin is consumed. The figure provides a schematic illustration of the process. Copper-tin intermetallic compounds are hard and brittle; at the surface they are easily oxidized. The oxidized intermetallic compound can adversely affect contact resistance and solderability.
Because the different types of tin plating are produced in different ways, with different thickness, their intermetallic compounds reach the metal surface at different times. Several studies have shown that, during the manufacturing process, Commercial Hot Dipped Tin quickly forms an intermetallic layer of 20-40-in thickness Thus, pockets of intermetallic have been found on rather "fresh" Commercial Hot Dipped Tin. Stringent connector performance requires thicker coatings.
With Air Leveled Tin, the tin is applied from a molten bath so there is an immediate intermetallic layer at the tin interface of about the same thickness as with Commercial Hot Dipped Tin. But, the air jets allow a thicker tin layer to be produced; the Air Leveled Tin surface has an abundance of residual ("pure") tin just after it is produced.
Because Electrotinned is not produced with hot molten tin, little or no intermetallic is formed at the copper-tin interface during the plating process. The structure of Electrotinned exhibits a finer grain size and tends to be more porous than the essentially "cast" structure of the "molten method" products. This affects the growth rate of the intermetallic. Reflow Tin starts as Electrotinned so there's essentially no intermetallic at this step. But the furnace treatment to melt the tin initiates a thin layer of intermetallic about the same thickness as that initially generated by the molten tin processes.
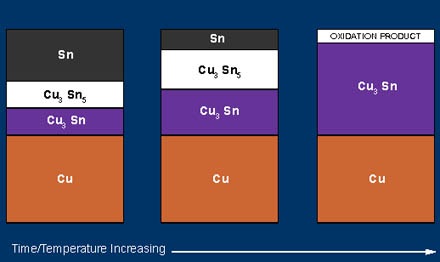 Figure 4 . Intermetallic Growth on Tin Coated Copper Alloy Strip
Figure 4 . Intermetallic Growth on Tin Coated Copper Alloy Strip