A most obvious requirement of copper conductors is that they transmit the designed electrical current without causing overheating. Resistive heating is a function of the square of the current times the resistance, while resistance is inversely proportional to cross-sectional area. A temperature rise above ambient is frequently allowed in connector design. It is important to realize that mildly elevated temperatures will cause several adverse consequences, which, while not individually serious, can have an additive effect. The adverse consequences include loss in strength of the connector alloy(usually represented by stress relaxation data), some dimensional changes which are reported using the coefficient of thermal expansion, an increase in electrical resistivity, and an increase in the growth rate of resistive oxides at a metal contact interface.
Resistive heating cannot be totally prevented by using high conductivity alloys. But knowing which alloys are high, intermediate, and low in conductivity lets the designer make rational choices in initial design. A brief discussion of conductivity will be given unde this topic heading and supplemented by more detailed information under other topic headings. The choices which result when tensile strength and conductivity must be balanced are illustrated in Figure 1, in which the three types of alloys (strengthened by solution, precipitation, and dispersion) are seen generally to occupy different regions when their strength is plotted against their conductivity. Solution-strengthened alloys occupy the intermediate strength, intermediate conductivity corner of the graph, while the dispersion-strengthened alloys tend to have intermediate strength and high conductivity and the precipitation-strengthened alloys have high strength and intermediate conductivity.
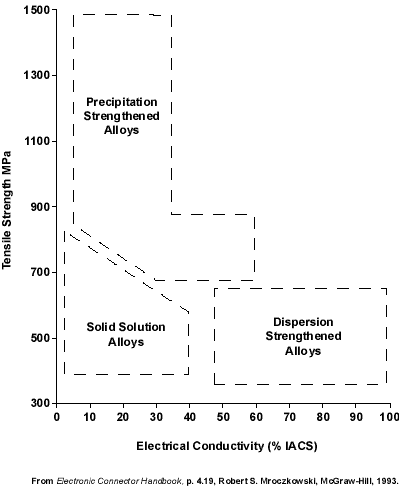 Figure 1. Typical ranges of electrical conductivity in alloys strengthened by different mechanisms.
Figure 1. Typical ranges of electrical conductivity in alloys strengthened by different mechanisms.Generally, the conductivity of copper is decreased when other metals are added, although differences among the additives can be dramatic. For example, the addition of silver has little effect, but phosphorous drastically reduces conductivity. Two factors should be kept in mind when considering the effect of alloying elements. First, the additives can often interact with one another to form compounds, which have been referred to as dispersed second phases. This may remove these elements from solid solution as they combine to form separate intermetallic compounds which have little effect on conductivity. For example, the deleterious effect of phosphorous is somewhat neutralized when it combines with oxygen. Second, precipitation-hardenable alloys exhibit higher conductivity in their hardened condition, the normal situation when they are in use, as a result of the combination of dissolved atoms to form discrete compounds.
Connector alloys, especially those which rely on solid-solution strengthening, are often used in the work-hardened condition. This is achieved by cold rolling of strip or drawing of wire. It is useful to know that this cold working slightly decreases electrical conductivity, and the loss of conductivity is generally proportional to the degree of deformation.
