The following are the thermal limitations of plating systems when nickel is used as an underplate:
| Cobalt Hardened Gold: | 125°C — Cobalt will diffuse to the surface at temperatures >125C |
| Nickel: | 150°C — Nickel will diffuse to the surface at temperatures >150C |
| Tin and its Alloys: | 105°C — Softening temperature |
| Palladium: | 200°C |
| Palladium Nickel: | 100°C |
| Gold Flash Palladium Nickel: | 125°C |
Relative to the cold side, all of the above materials may be used at -65°C except for tin and its alloys which should be limited to -40°C.
If nickel is not used as an under-plate, copper diffusion can occur quickly when gold is used. The following table illustrates time at temperature which would result in unstable resistance levels being reached.
| 100 micro-inch Au over Copper | 100 micro-inch Au over Nickel | |
|---|---|---|
| 100°C | ≈ 30 Days | No Evidence |
| 125°C | 10 Days | No Evidence |
| 150°C | 2 Hours | >100 Days |
For tin (alloys), the problem is intermetallic formation. The figure below illustrates time/temperature realtionship and intermatallic thickness. The intermatallic is resistive. Thus if 100 µinch of tin is plated over a substrate, in less than 2 years at 50°C, the total coating will be intermetallic and will cease to function.
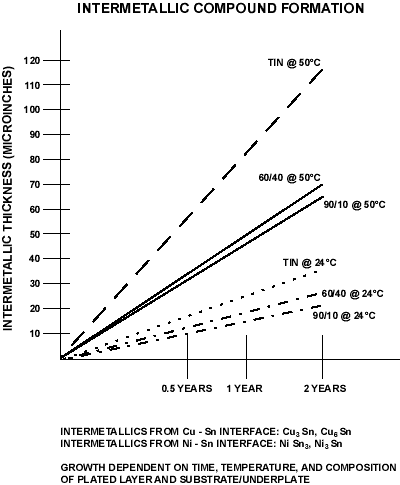 Figure 8. Intermetallic Compound Formation
Figure 8. Intermetallic Compound Formation