Understanding nickel, copper and tin oxide formation is important to put in proper perspective when considering functional limitations of connectors. The figure below is a set of plots illustrating film growth over time and film thickness vs. resistance increase.
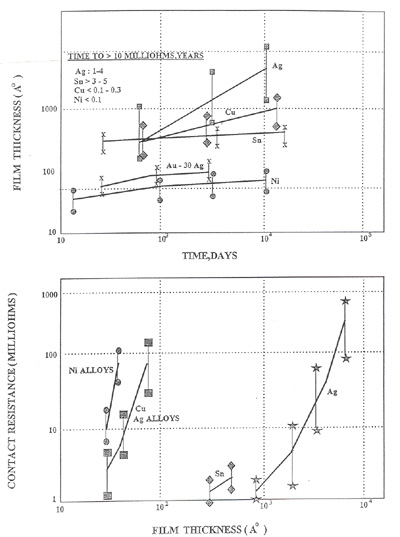 Figure 4. Contact Resistance as a Function of Film Thickness
Figure 4. Contact Resistance as a Function of Film ThicknessThis figure illustrates the following points:
- The film/oxide formation occurs very quickly.
- Nickel and tin oxides are self-limiting. That is, they will form to a given thickness and will, in essence, reach equilibrium over time.
- Copper oxides will form quickly and continue to grow over time.
- Relatively thin copper and nickel oxides will result in unstable resistance. Thick tin oxides will also result in unstable resistance.
If unstable resistance change is defined ≥ 50.0 milli-ohms, the following oxide thickness can result in said changes. Many specifications allow for only a 10 milli-ohms change which means that even thinner layers will be disruptive.
| Nickel oxide | ≈ | 60A (<1.0 micro-inch) |
| Copper oxide | ≈ | 75A (<1.0 micro-inch) |
| Tin oxide | ≈ | 5000A (<20.0 microinch) |
| where A = 3.937 x 10-9 inch | ||
Thus thin layers of base oxidation can result in unacceptable signal loss. In the presence of a polluted environment, additional complex compounds can form especially when C12, H2S, NO2 and SO2 are present at the same time. Although normally found in very low concentrations (ppb), their interaction may be significant. The following generalizations apply to the basic plated surfaces in the presence of a polluted atmosphere.
Gold — Pore free gold will not react unless base metal porosity exist. If base metal porosity exist, the pore may become active (see porosity section) and base metal corrosion products will exit through the pore site and "creep" across the gold surface.
Gold Flash Pd Ni — If the gold flash is porous or is worn out due to the number of mating cycles thus exposing Pd Ni to the environment, reactions will occur particularly when SO2 is present.
Tin (alloys) — Due to the softness of tin and assuming appropriate normal forces are used, this material may react but said oxides are easily displaced and are not a problem unless base metal exposure occurs or thick layers of copper/tin intermetallic exist.
In essence, applications where option card capabilities are of primary concern, reactions to harsh environments must be considered. In this instance, the connector system may be subjected to several mating cycles and then sit in the equipment for several months unmated directly exposing the contact surfaces to the environment (particularly severe on plug connectors). Of particular concern is gold plating on blade contacts or square posts with gold plating. Here, the plating tends to thin out at the corners or edges making these areas susceptible to the corrosion process.
