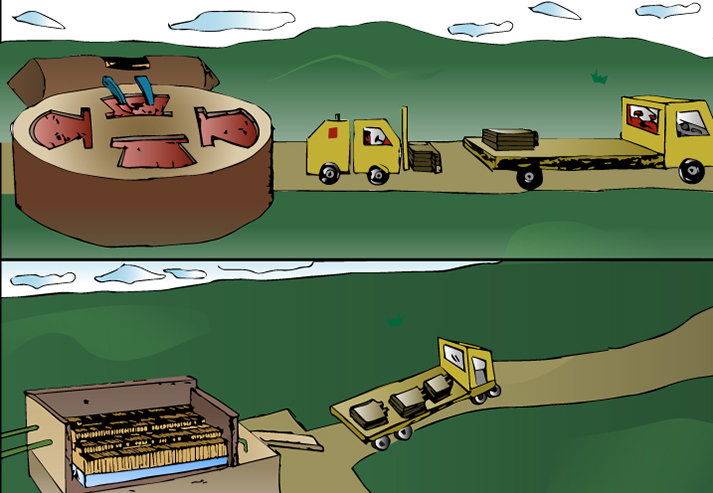
 Top: Copper from smelter is cast as anodes. Bottom: Electrowinning produces copper cathodes ready for shipment.
Top: Copper from smelter is cast as anodes. Bottom: Electrowinning produces copper cathodes ready for shipment.Sulfide Ores: After smelting, the 99% copper rich material is poured into molds as "anodes" using a casting wheel and transported to the plating house. In this form, they are ready for the next step, which involves dissolving and re-plating the copper to increase its purity level.
Oxide Ores: The copper-bearing solution, from the solvent extraction operations, is plated into pure copper cathodes using a process called electrowinning. Stainless steel blanks are added to the plating tanks to act as cathodes and copper is plated onto them by electro-chemical deposition. It takes about a week before the cathode is ready to be removed from the tank so the copper can be stripped off the blank. The cathodes are now 99.99% pure copper and ready to be made into wire, tube or any number of useful products.